Why can't saltwater fish live in freshwater and vice versa?
 Saltwater fish can't survive in freshwater because their bodies are highly concentrated of salt solution (too much for freshwater). The water would flow into their body until all their cells accumulate so much water that they bloat and die eventually.
Saltwater fish can't survive in freshwater because their bodies are highly concentrated of salt solution (too much for freshwater). The water would flow into their body until all their cells accumulate so much water that they bloat and die eventually.
On the other hand, freshwater fish can't survive in the ocean or saltwater because the seawater is too salty for them. The water inside their bodies would flow out their cells, and they wiil die of dehydration.
Both processes are called Osmosis. But how exactly this process happens and why?
What is Osmosis?
 Before knowing the process on why saltwater fish can't live in freshwater and freshwater fish can't live in the ocean, you must first understand the process of osmosis. Osmosis is the movement of liquid molecules through a semipermeable membrane (like the thin film inside of an egg) from a low concentrated solute to a high concentrated solute.
Before knowing the process on why saltwater fish can't live in freshwater and freshwater fish can't live in the ocean, you must first understand the process of osmosis. Osmosis is the movement of liquid molecules through a semipermeable membrane (like the thin film inside of an egg) from a low concentrated solute to a high concentrated solute.
A semipermeable membrane is a barrier that has small holes big enough to let liquid molecules enter, but small enough to block the flow of salt, sugar or other concentrated content. The membrane of a cell is semipermeable which means it blocks larger molecules and lets smaller molecules (like water molecules) pass through.
For example, if you try submerging a raisin in fresh water, it will swell up; this is because the raisin has higher sugar concentration than the freshwater and water will keep flowing in. But, if you put a raisin in supersaturated salt water, the raisin shrinks even more because the water flows out. The supersaturated salt water has higher content concentration than the raisin.
Osmotic Pressure and Tonicity
 Osmotic pressure is the applied pressure to counteract the flow of water molecules across the semipermeable membrane. As water moves across a semipermeable membrane to a higher concentrated solution, the osmotic pressure increases. If the solution concentration is equal on both sides of the membrane, there is no need for water to move across. Therefore, no osmotic pressure takes place.
Osmotic pressure is the applied pressure to counteract the flow of water molecules across the semipermeable membrane. As water moves across a semipermeable membrane to a higher concentrated solution, the osmotic pressure increases. If the solution concentration is equal on both sides of the membrane, there is no need for water to move across. Therefore, no osmotic pressure takes place.
Tonicity is the measure of osmotic pressure. There are three classifications of tonicity that you should know to understand better why fishes only survive in specific bodies of water. The three classifications of tonicity are:
- Hypertonicity
- Isotonicity
- Hypotonicity
What are Hypertonic, Hypotonic, and Isotonic
 Let us use cells for example.
Let us use cells for example.
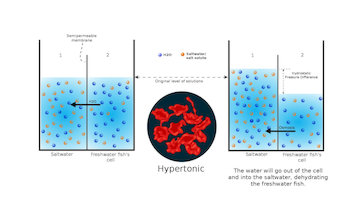
Hypertonicity is caused by a higher content concentration outside the cell than inside of it, and this results in the cell shrinking because water flows out of the cell to dilute the content outside.
Hypotonicity, on the other hand, is caused by the high content concentration inside the cell, making the cell bloat and burst because water keeps flowing in to dilute the cell's solution.
Isotonicity is the balance of solute concentration inside and outside the cell. There is no change in cell volume and no movement of water between the membrane. One example for isotonicity is red blood cells in plasma solution.
Do fishes drink water? Do fishes pee?
Yes! Due to osmosis, fishes need to osmoregulate, the process of maintaining a right amount of water in their bodies. Some fishes need to drink water, but all have to urinate frequently. Freshwater fish and saltwater fish survive according to how much salinity their body can sustain.
Seawater is hypertonic to the fishes living in the ocean, which means that water is continually being sucked out of their bodies. To survive, saltwater fishes continually drink lots of water to compensate for water loss caused by osmosis. They filter out excess salt from their bloodstream through their gills and kidneys by urinating.
For the freshwater fish, they don't need to drink water, but they do have to urinate. Because freshwater is hypotonic to the fishes living in it, water is continually entering their bodies through their gills, skin, or their mouths when they eat. To balance the amount of water in their bodies, all they have to do is urinate frequently.
Wait! What? Does it mean than seawater fishes just have to pee more to live in freshwater?
Yes, but no! The cells of the seawater fish will be saturated faster than it is able to pee (or faster than its kidneys can handle).
Fresh and salty water fishes
 However, there are certain species of fishes who can survive in both freshwater and saltwater. The species of fish, called euryhaline fish, can tolerate and migrate in both bodies of water at or for a certain amount of time. There are two types of euryhaline fish: anadromous and catadromous.
However, there are certain species of fishes who can survive in both freshwater and saltwater. The species of fish, called euryhaline fish, can tolerate and migrate in both bodies of water at or for a certain amount of time. There are two types of euryhaline fish: anadromous and catadromous.
Anadromous fishes are born in freshwater, but spend most of their lives in the ocean, only returning to freshwater to lay their eggs. Examples of these fishes include striped bass, sturgeon, smelt, and salmon.
Catadromous fishes, on the other hand, live in freshwater but have to enter the sea through rivers to spawn. An example of a catadromous fish is the European Eel.
Saltwater Fish vs Freshwater Fish Researches and References
If you want to know more about saltwater and freshwater fishes, take a look at some of our references below.
Images we use are from Wikimedia commons, Patrice Laborda, Paul Cowell, and the Osmosis Draws and montage are from Marrielle Ferrer.
- Osmosis
- Osmosis Definition in Chemistry
- Tonicity
- Osmotic pressure
- Osmotic Pressure and Tonicity
- Why Can't Freshwater Fish Survive in Saltwater and Vice Versa?
- Can Saltwater Fish Live in Fresh Water?
Speaking about fish, now I want to dive...
Last, but not Least
If you would like to receive interesting content like this in your email Inbox, subscribe to our newsletter.
In addition to our monthly newsletter, we will send you our weekly e-Bulletin with one fascinating topic, like today's article above. There will be no advertising nor sales pitch.
As always we want to thank Youtube and Wikipedia commons for some amazing images and/or videos on this page!
Thanks for reading, and if you wish, see you next week!
The Research and Media Team at Scotty's.
More Pictures About Saltwater and Freshwater Fishes





































